Animal Husbandry Archives
First Report of Molecular-Genetic Description and Barcoding of Hard Ticks (Metastigmata: Ixodidae) Distributed in Georgia
Mariam Zakalashvili*, Nino Abashidze, Gvantsa Chanturia, Giorgi Chakhunashvili, Julieta Manveliani, Giorgi Tomashvili, Roena Sukhiashvili, David Tsereteli and Paata Imnadze
Department of Virology and Molecular Biology, National Center for Disease Control and Public Health (NCDC), 99 Kakheti Hwy, 0198, Tbilisi, Georgia
Cite this as
Zakalashvili M, Abashidze N, Chanturia G, Chakhunashvili G, Manveliani J, Tomashvili G, et al. First Report of Molecular-Genetic Description and Barcoding of Hard Ticks (Metastigmata: Ixodidae) Distributed in Georgia. Animal Husbandry Arch. 2025;1(1):005-013. Available from: 10.17352/aha.000002Copyright Licence
© 2025 Zakalashvili M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Hard ticks (Ixodidae) are important vectors of infectious diseases worldwide. Because of its geographical location, Georgia is a bridge between different biogeographical and climate zones and is rich in biodiversity. Different species of hard ticks are distributed in Georgia and have not yet been barcoded. Understanding the spatial distribution of disease vectors is important for health risk assessment. Cases of tick-borne diseases (borreliosis, Crimean-Congo hemorrhagic fever) have also been reported. In this study, we perform molecular-genetic characterization and barcoding of hard ticks distributed in Georgia. Mitochondrial cytochrome C oxidase I (COI-5P) was the molecular marker. Samples of hard ticks were collected from different regions of Georgia (Khashuri, Ambrolauri, Aspindza, Akhaltsikhe), DNA extraction and PCR amplification protocols were selected and optimized; Specimen were sequenced with the Sanger sequencing method and results were analyzed in Geneious Prime. All sample species were identified with high reliability (average query coverage 99.83 %, average percent Identity 99.82 %): Hyalomma scupense (=detritum), Hyalomma marginatum, Dermacentor marginatus, Rhipicephalus annulatus, and Rhipicephalus bursa. All of the listed species pose a threat to human health (including Crimean-Congo hemorrhagic fever, etc.), except for the species Rhipicephalus annulatus, which mostly infects cattle. The sequences are uploaded and published in GenBank and BOLD (Barcode of Life Data Systems), with indications of the samples’ geographical locations. To our knowledge, this is the first DNA barcoding results of hard ticks distributed in Georgia. It allows further phylogenetic analysis and comparison of species distribution across the Caucasus region and the globe. Future research is important to collect and analyze Ixodidae samples from all regions of Georgia in order to obtain more comprehensive data.
Background
Ticks are important disease vectors globally and in Georgia, as they transmit different types of pathogenic bacteria, protozoa, and viruses. Compared to other arthropod vectors, ticks have the ability to spread a greater variety of pathogens [1]. They are widespread organisms and can be found anywhere, especially in warm and humid climates [2]. Feeding on the blood of birds, mammals, reptiles, and sometimes amphibians, ticks pose a threat to human and animal health and infest livestock which often causes economic losses [3,4].
The order of ticks Metastigmata (=Ixodida) contains three families: hard ticks (Ixodidae), soft ticks (Argasidae), and Nuttalliellidae [2,5]. Among those three, hard ticks are the vectors of the most serious zoonotic diseases. This family includes 14 genera and more than 700 species [6].
Hard tick barcoding is becoming increasingly important due to the rising number of tick-borne diseases being reported in the country, including lyme disease, Crimean-Congo hemorrhagic fever, tick-borne relapsing fever, and tick-borne encephalitis [7], this growing prevalence underscores the urgent need for barcoding which can lead to more sophisticated preventive measures to address these critical public health challenges.
Molecular studies on tick identification are limited in Georgia, however, morphological data can be found in the fourth volume of the US Department of Agriculture catalog that describes the world distribution of ticks. According to the catalog, 7 genera (Boophilus, Dermacentor, Haemaphysalis, Hyalomma, Ixodes, Ornithodoros, Rhipicephalus) and 40 species of hard ticks were distributed in the territories of Georgia [8].
Morphological identification of Ixodid ticks collected in the North Caucasus in 2018 describes 5 genera: Dermacentor, Haemaphysalis, Hyalomma, Ixodes, and Rhipicephalus [9]. The territories studied in the article border Georgia from the north and therefore can prove informative for studying Ixodidae distribution dynamics in Georgia and the South Caucasus. Two genera mentioned in the Maryland catalog (Boophilus and Ornithodoros) are not monitored in the North Caucasus. The article also suggests that Ixodida ghilarovi is endemic to the Greater Caucasus and also found in Georgia; Species I. arboricola, I. caledonicus, and I. unicavatus are reported to be found in South Caucasus and probably spreading to the north [9]. According to the European Center for Disease Prevention and Control, Hyalomma marginatum (last monitored in March 2021), and Rhipicephalus bursa (last monitored in July 2019) have been recorded in Georgia [10].
DNA barcoding is a commonly used method for tick species identification along with morphological discrimination [11]. Molecular methods are particularly significant when study samples are fragmental or of nymphal or larval stages [12]. The cytochrome C oxidase I subunit (COI) gene of the mitochondrial genome is widely used as a molecular marker for tick barcoding [13,14]. In a study of 26 hard tick species, BIN (Barcode Index Numbers) analysis showed that within species, the average maximum deviation is 1.59%, and the average deviation among nearest neighbors is 12.8%. This indicates a plausible “barcode interval” [12]. In a 2018 study, based on studies of 8 genera of hard ticks common in Australia, it was determined that among the molecular barcodes COI, ITS2, 16S, and 12S regions, the COI gene is the most efficient for species barcoding [15].
DNA barcoding of ticks allows us to register them in international databases, with indications of their geographical locations. As a result, the risks of the spread of infectious agents important to humans and animals are evaluated, which forms the basis for determining appropriate preventive measures [16]. In addition, barcoding provides a global picture of species distribution, aiding phylogenetic and evolutionary studies [17]. One such database is the Barcode of Life Data System – BOLD, created by the Genomics Biodiversity Center (Canada) where data is stored and analyzed [18]. The aim of this research was to identify species of hard ticks through the DNA barcoding method, using the COI-5P gene as a molecular marker, and to deposit the results at BOLD, with indications of the samples’ geographical locations.
Materials and methods
Sample collection and DNA purification
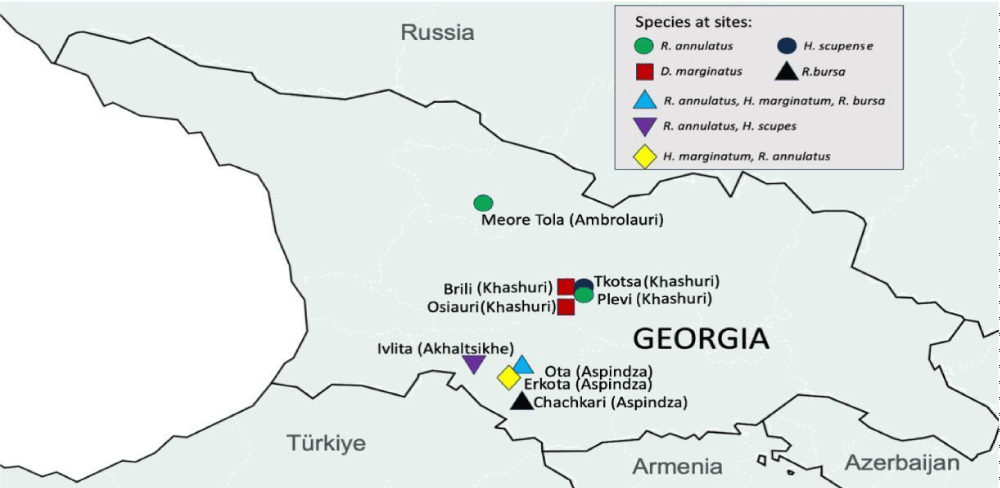
Hard tick samples were collected from different parts of Georgia during 2018-2021 using the flagging/dragging method or hand-picked from cattle. Geographical locations were: Khashuri, Aspindza, Akhaltsikhe, and Ambrolauri. The tick samples used in this study were collected in full compliance with the ethical norms and guidelines of Georgia, adhering to the internal regulations of the National Center for Disease Control and Public Health (NCDC).
Samples were identified morphologically according to the Ticks of Europe and North Africa: A Guide to Species Identification 1st ed. 2017 Edition [19], after which tick legs were stored in 80% ethanol at -20 0C and used for DNA purification and downstream molecular analyses.
Collected samples were identified morphologically and assigned into three different genera (Rhipicephalinae, Hyalomminae, and Dermacentor) and five species (Hyalomma scupense, Hyalomma marginatum, Rhipicephalus annulatus, Rhipicephalus bursa, Dermacentor marginatus). However, species-level identification was difficult in several samples. 41 samples representing all morphologically identified specimens (Khashuri, Aspindza, Akhaltsikhe, and Ambrolauri), were then chosen for DNA barcoding of the COI-5P region. Table 1 depicts these samples in detail.
gDNA was extracted with an OxMag Soft Tissue DNA Purification kit (OxGEn, Georgia). The extraction protocol was optimized for easy homogenization: Before DNA extraction, samples were washed with 200 μl of 1x PBS solution. Each sample was mixed with 20 μl of lysis buffer and 5 μl of proteinase K (20 mg/ml) and vortexed for 15 sec. In the next step, the samples were finely crushed/homogenized with a lancet; 160 μl of Solution A and 15 μl of proteinase K were added, vortexed for 2 min, and incubated in a 56 0C thermoblock overnight. The rest was conducted according to the manufacturer’s instructions for the OxMag Soft Tissue DNA Purification kit.
PCR amplification
Two pairs of primers were tested for COI-5P PCR amplification: LCO1490/HCO2198 (forward GGTCACAAATCATAAAGATATTGG, reverse TAAACTTCAGGGTGACCAAAAAATCA-3’) [20] and ChelF1/ChelR2 (forward CCTCCTCCTGAAGGGTCAAAAAATGA, reverse GGATGGCCAAAAAATCAAAATAAATG-3’) [21]. ChelF1/ChelR2 primers were chosen for further research as they showed more sensitivity in amplification reactions. PCR reactions were performed in 25 µl consisting of 1 ng of the DNA sample, 1.25 U of Taq DNA Polymerase (OxGEn, Georgia) and its reagents: 2.5 mM of MgCl2, 2.5 µl of 10x Buffer; 200 µM of each dNTP and 100 nM of each primer. PCR cycling conditions were as follows: Initial denaturation at 95 0C for 5 min; 35 cycles of 95 0C for 60 sec, 50 0C for 90 sec, 72 0C for 90 sec; final extension at 72 0C for 10 min. PCR reactions were conducted in the BIOER GeneExplorer thermocycler (model GE-96G). Results were analyzed on 1% agarose gel, TBE buffer, and GelGreen gel dye (Sigma-Aldrich), visualized on SmartBlue (Accuris) transilluminator. NEB 1 kb DNA Ladder was used to assess amplicon lengths. Subsequently, PCR fragments were purified with 1x OxMag XP beads (OxGEn, Georgia).
Sequencing and bioinformatical analysis
Purified amplicons were sequenced in Macrogen, Amsterdam on ABI PRISM 3730XL Analyzer (Applied Biosystems) with BigDye Terminator v3.1 Cycle Sequencing kit (Applied Biosystems). Sequences were analyzed in Geneious Prime (version 2022 1.1) and consensus sequences were generated. NCBI BLAST (Megablast – fast, high similarity matches) was used for species identification [22]. To verify the mitochondrial origin of the sequences and exclude potential nuclear mitochondrial DNA sequences (numts), we conducted a BLASTn search against both the NCBI mitochondrial genome database and available nuclear genome assemblies of related species. The sequences were compared to known COI mitochondrial genes and we could not detect any numbers. Additionally, the tick samples were uploaded to the Barcode of Life Data System (BOLD), where each sequence was assigned to its relevant Barcode Index Number (BIN).
The MUSCLE alignment tool was used to process the consensus sequences. To investigate the evolutionary relationships among the tick species, we constructed phylogenetic trees using three distinct methods: (a) Geneious Tree Builder (Neighbour-joining), (b) RAxML (Maximum Likelihood), and (C) MrBayes (Bayesian Inference) [23,24].
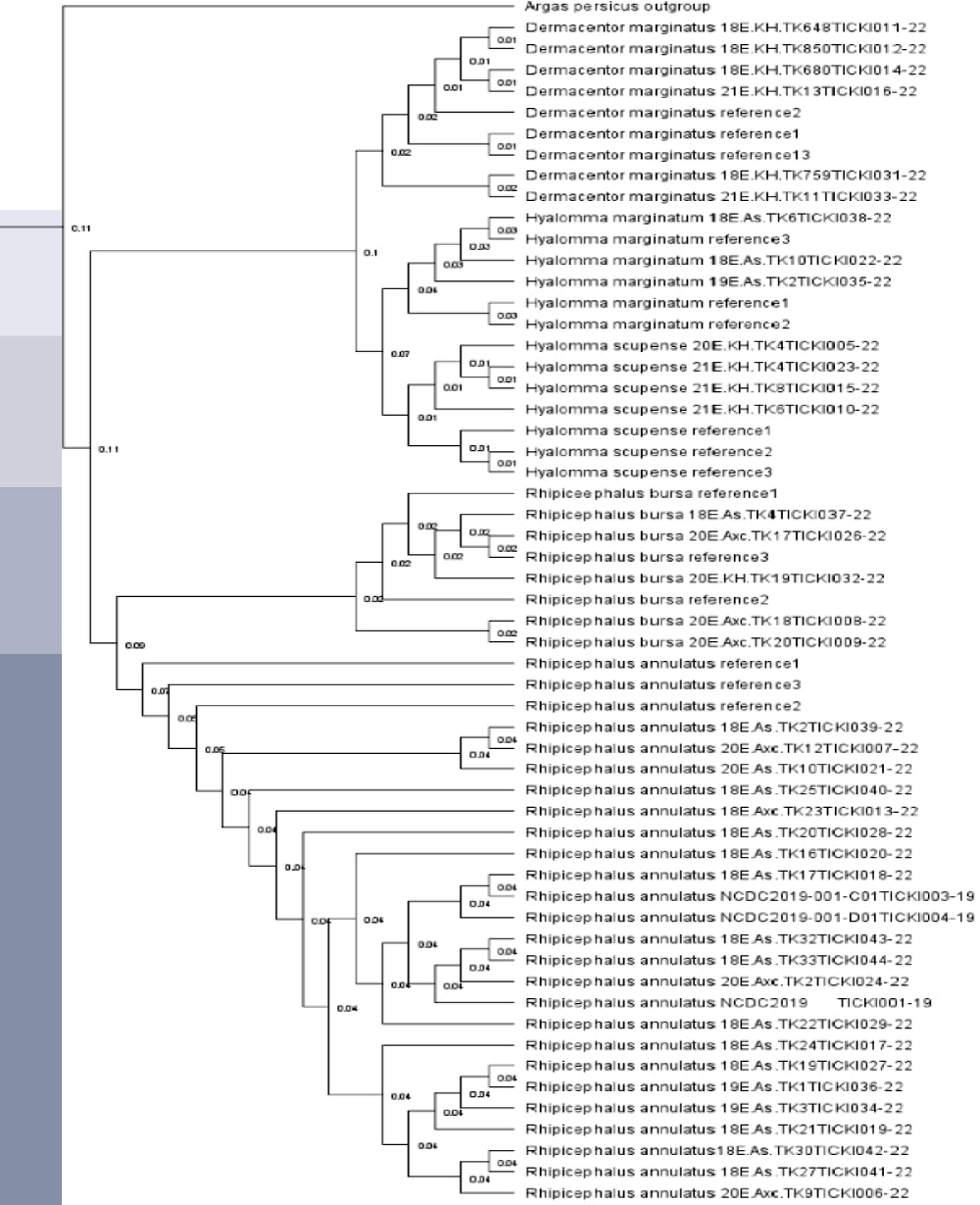
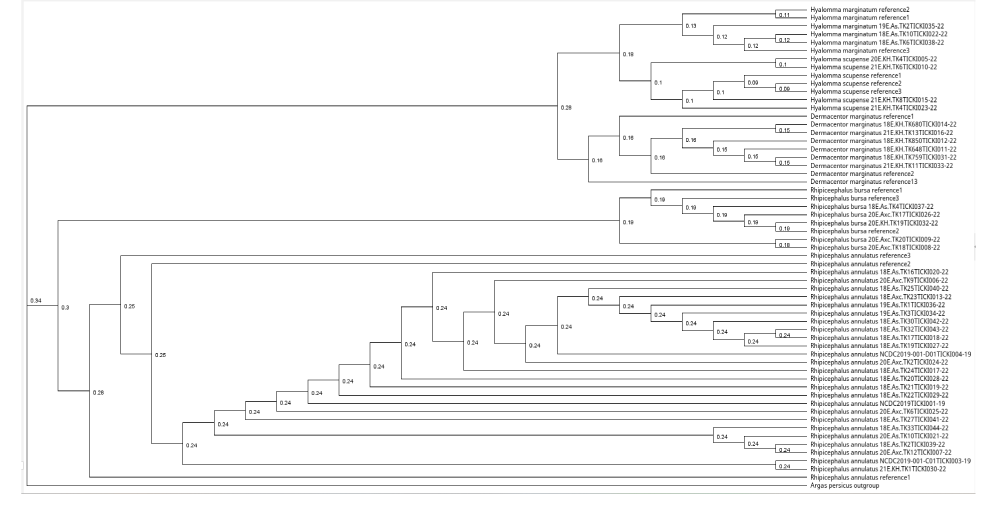
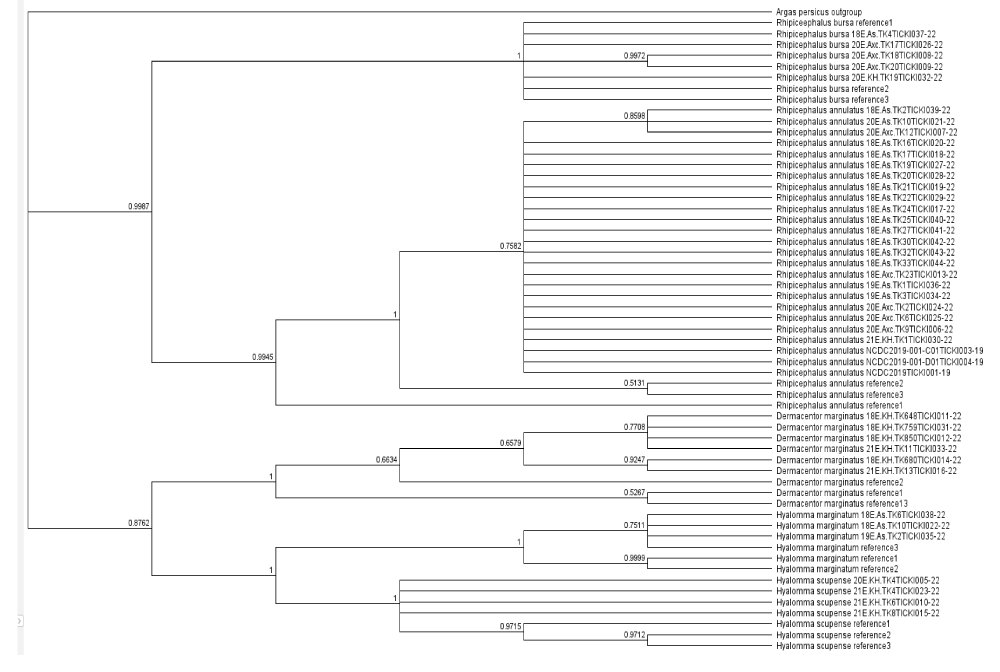
The phylogenetic tree was constructed using the Geneious Tree Builder software, employing the Neighbour-joining method. Argas persicus was used as an outgroup to root the tree. Three reference sequences for each tick species were downloaded from NCBI GenBank. This method allowed for a quick visualization of the phylogenetic relationships based on genetic distance. (b) The second tree was generated using RAxML version 8.2.11, applying the Maximum Likelihood (ML) method with the General Time Reversible (GTR) + GAMMA substitution model. To evaluate the reliability of the branches, 1,000 bootstrap replicates were performed. The analysis was initiated from a completely random tree, with the convergence criterion set to ensure accurate results. Sequence data were partitioned, and custom parameters were applied according to the specifications in RAxML. (c) A phylogenetic tree was conducted using the Bayesian inference (BI) approach (MrBayes version 3.2.6). The analysis was performed using the HKY85 substitution model, the tree was rooted with Argas persicus as the outgroup. The posterior probabilities were calculated to assess the reliability of the inferred phylogeny.
A phylogenetic tree was constructed in Geneious Tree Builder with the Neighbour-joining method. For each species, three reference sequences were downloaded from NCBI Genomic Bank, and Argas persicus was used as an outgroup. The samples were uploaded to the international database BOLD - Barcode of Life Data System and each sequence was assigned to the relevant BIN. The phylogenetic tree for the tick samples was constructed using RAxML version 8.2.11 with the Maximum Likelihood (ML) method, applying the GTR + GAMMA substitution model. The analysis was conducted with the “rapid hill-climbing” algorithm, which is designed to quickly estimate the ML tree topology. A total of 1,000 bootstrap replicates were used to evaluate the reliability of the tree’s branches. The tree search started from a complete random tree, with the convergence criterion set for the ML search to ensure accuracy. Sequence data were partitioned accordingly, and the analysis was performed with additional custom parameters as specified in the RAxML software. The resulting tree was rooted using the outgroup species Argas persicus. All reference sequences for the tick species were downloaded from NCBI GenBank, and species identified in this study were labeled with their specific codes, which were uploaded to the Barcode of Life Database (BOLD). The phylogenetic tree for the tick samples was constructed using the MrBayes version 3.2.6 software, employing a Bayesian inference (BI) approach. The analysis was conducted with the HKY85 substitution model, incorporating rate variation across sites using a gamma distribution with 4 gamma categories. The tree was rooted with Argas persicus as the outgroup. MCMC (Markov Chain Monte Carlo) settings included a chain length of 1,100,000, with subsampling every 200 generations. The analysis was conducted with 4 heated chains and a burn-in length of 100,000 to ensure convergence and reliable estimation of the tree topology. The heated chain temperature was set to 0.2, and a random seed of 16,281 was used for the analysis. The molecular clock was applied with uniform branch lengths, and priors were set for unconstrained branch lengths using a GammaDir distribution (1, 0.1, 1, 1). The shape parameter was set to an exponential distribution with a value of 10. Reference sequences for the species were downloaded from NCBI GenBank, and species identified in this study were labeled with their specific codes, which were uploaded to the Barcode of Life Database (BOLD). The tree’s posterior probabilities were assessed to evaluate the reliability of the inferred phylogeny. If the results from this study are used, the appropriate citation for MrBayes (Huelsenbeck & Ronquist, 2001) should be included.
Results
We obtained the first DNA sequencing results of ticks distributed in Georgia (Figure 1). In this study, the COI gene sequences for species within the Ixodidae family (hard ticks) exhibited both conserved and variable regions. The gene matrix was highly conserved, with 74.62% of the sequences being conserved across the samples, 25.38% of the sequences showed mutations, which were primarily located in the variable regions of the gene, providing the necessary variation for species identification. The lengths of the COI sequences varied between 497 bp and 695 bp, the variation in length reflects natural diversity within the species, BLASTn analysis confirmed that all COI sequences aligned exclusively to mitochondrial genomes, with no significant matches to nuclear genomic regions. No evidence of numts was detected, ensuring that only authentic mitochondrial sequences were included in the study.
All sample species were identified with high reliability (Query coverage above 99.3 %) by NCBI BLAST megablast, and assigned to relevant BINs by BIN-RESL algorithm. 6 samples collected from Khashuri were identified as Dermacentor marginatus by NCBI BLAST, and assigned to BIN BOLD:AAL1447. 3 samples from Aspindza were identified as Hyalomma marginatum (BOLD:AAC1486). 4 samples were identified as Hyalomma scupense (=detritum) from Khashuri and Akhaltsikhe (BOLD:AAF1229). 23 samples were Rhiphicephalus annulatus from Ambrolauri, Akhaltsikhe, and Aspindza, (BOLD:AAY0311). 5 samples from Akhaltsikhe and Aspindza were identified as Rhiphicephalus bursa (BIN BOLD:ADB0344) (Table 2). Morphological identification revealed ~86 % accuracy to molecular results.
Four BINs comprise of more than one species. Those bins are BOLD:AAL1447 (D. marginatus, D. silvarum), BOLD:AAC1486 (H. marginatum, H. turanicum, H. rufipes), BOLD:AAF1229 (H. detritum, H. scupense), BOLD:AAY0311 (R. anatolicum, R. annulatus). BIN BOLD:ADB0344 consists of a single species: Rhiphicephalus bursa.
BOLD Aligner (amino acid-based HMM) Distance to nearest neighbor is 10.99 % between Hyalomma scupense and Hyalomma marginatum; 12.06 % between Rhiphicephalus annulatus and Rhiphicephalus bursa; 17.75 % between Dermacentor marginatus and Rhiphicephalus annulatus. Mean intra-specific values range from zero (in the case of Hyalomma scupense) to 9.45% (Rhiphicephalus annulatus).
The Results are published in NCBI GenBank and BOLD Dataset. Each sample has its indications of the geographical location where it was collected, the date of its collection, and its unique codes [25].
Discussion
The objective of this study was to barcode the ticks distributed in Georgia utilizing the COI-5P gene as a molecular marker. Tick barcoding is a cornerstone for future disease management as it enables accurate species identification, aiding in tracking and controlling tick-borne disease risks. It is noteworthy that in several samples morphological identification was problematic up to the species level and the results showed 86 % accuracy in molecular analysis. Therefore, our study demonstrates the relevance of the DNA barcoding method and suggests it be used along with traditional morphological analysis, as recommended in other phylogenetic studies [26,27]. Sample homogenization steps proved essential for successful gDNA extraction and PCR amplification. This can be explained by the thick exoskeleton of hard tick leg samples that need crushing/cutting [28]. A comparison of COI-5P amplification with two different primer pairs revealed that ChelF1/ChelR2 obtained better results than LCO1490/HCO2198 primers.
High inter-specific variability of cytochrome C oxidase I subunit proved reliable for identifying species with high percent identity in the NCBI database and assigning to relevant BINs in BOLD. The abundance of reference specimens also facilitates phylogenetic analysis of COI-5P gene sequences [29]. We hope this study will provide a basis for the establishment of a molecular data platform for the Ixodidae of the Georgian fauna.
The phylogenetic analyses conducted using Bayesian, Maximum Likelihood, and Neighbor-Joining methods (Figures 2-4) consistently revealed distinct genetic clustering patterns among the tick species, highlighting their evolutionary relationships. All three methods corroborated the outgroup positioning of Argas persicus as a distant root, reinforcing its evolutionary role. The tight clustering observed within species such as Rhipicephalus bursa and Hyalomma scupense suggests a close genetic relationship among samples, with minimal genetic variation, supporting the idea of limited genetic diversity within these groups. However, slight variations in genetic distances, particularly in Rhipicephalus annulatus, indicate some level of intraspecific genetic variability, which could reflect different populations or environmental influences.
Notably, the Bayesian method provided additional insights into genetic diversity within certain species, such as Rhipicephalus annulatus and Dermacentor marginatus, where some samples exhibited larger genetic distances. This variability, while still within the general clustering pattern, may suggest underlying evolutionary dynamics or historical diversification within these species. Overall, the comparative analysis of these methods underscores the robustness of the phylogenetic tree and offers valuable perspectives on the genetic structure of tick populations, highlighting both the tight genetic relationships within species and the distinct genetic separation between them. These findings contribute to a deeper understanding of tick species’ evolutionary patterns and have implications for future studies on tick biology and vector-borne diseases.
All of the 5 species identified in this study are vectors of zoonotic diseases and pose a threat to humans as well as animals. H.scupense, H.marginatum, D.marginatus, and R.bursa transmit Crimean-Congo hemorrhagic fever virus and other pathogens such as Theileria, Anaplasma, Babesia, etc. [30-33]. Rhiphicephalus annulatus is the only species of those five that does not infest humans but causes damage to cattle by transmitting Babesia bovis, Babesia bigemina, and Anaplasma marginale [34](Table 3). While previous studies dating back to the 1970s in Georgia have explored the vector potential of ticks [8], the current findings presented in this research offer novel DNA barcoding data for Ixodidae. All of the described species are present in the Maryland catalog [8], as well as other species of respective genera. Two genera: Ornithodoros and Boophilus (with representatives O. alactagalis and B. calcaratus) are mentioned in the catalog but were not identified in our study. Our results are in agreement with the research from different neignbouring countries of Georgia. - Hyalomma marginatum is widely distributed across the Caucasus including countries such as Armenia, Azerbaijan, and Georgia (Yun, et al. 2015). Dermacentor marginatus is found in Turkey - It has also been recorded in the Caucasus region, which includes parts of Russia, Armenia, and Azerbaijan. Rhipicephalus annulatus, and Rhipicephalus bursa: - are also found in the Caucasus region, including parts of Russia, Armenia, and Azerbaijan (Orlova, 2023).
To our knowledge, this is the first report from Georgia and all species identified in this study are vectors of zoonotic diseases and pose a threat to humans as well as animals. This study is constrained by the selected genetic marker COI-5, which may not capture full genomic variability. Additionally, the size of the dataset limits resolution, affecting species differentiation and evolutionary insights. In the future, we plan to have a more comprehensive sample size and analyze samples with different genetic markers and sequencing techniques.
Conclusion
This study is the first report of hard tick (Ixodidae) DNA barcoding in Georgia. We collected samples from different parts of Georgia (Khashuri, Aspindza, Akhaltsikhe, Ambrolauri), and after morphological identification, sequenced the COI-5P gene of 41 samples for DNA barcoding. 5 different species of hard ticks were identified and published in the international database BOLD: Hyalomma scupense (=detritum), Hyalomma marginatum, Dermacentor marginatus, Rhipicephalus annulatus, and Rhipicephalus bursa. All of them are potential threats to human and animal health as they transmit CCHF, Rickettsia, Anaplasma, Babesia, and other pathogens. While cases of tick-borne diseases are being reported in the country, the data of Ixodidae DNA barcoding is important for health risk analysis and for monitoring the distribution of vectors.
We would like to express our sincere gratitude to OxGEn Solutions LLC for kindly providing reagents and funding for our research.
Authors’ contributions
GvC and PI provided project supervision and GiC project planning. JM collected and classified specimens. RS assisted with DNA extraction and PCR. DT participated in searching for articles. MZ and NA conducted molecular work and wrote the manuscript. GT participated in data analysis. All authors approved the final manuscript and consented to publication.
Availability of data and materials: Voucher specimens are available as described in the text and sequence data are posted on BOLD and GenBank.
- Jongejan F, Uilenberg G. The global importance of ticks. Parasitology. 2004;129 Suppl:S3-14. Available from: https://doi.org/10.1017/s0031182004005967
- Anderson JF. The natural history of ticks. Med Clin North Am. 2002;86(2):205-18. Available from: https://doi.org/10.1016/s0025-7125(03)00083-x
- Esteve-Gassent MD, Castro-Arellano I, Feria-Arroyo TP, Patino R, Li AY, Medina RF, et al. Translating ecology, physiology, biochemistry, and population genetics research to meet the challenge of tick and tick-borne diseases in North America. Arch Insect Biochem Physiol. 2016;92(1):38-64. Available from: https://doi.org/10.1002/arch.21327
- Barnes RD. Arthropod. Britannica. 2021. Available from: https://www.britannica.com/animal/arthropod
- Horak IG, Camicas JL, Keirans JE. The Argasidae, Ixodidae and Nuttalliellidae (Acari: Ixodida): a world list of valid tick names. Exp Appl Acarol. 2002;28(1-4):27-54. Available from: https://doi.org/10.1023/a:1025381712339
- Nava S, Venzal JM, González-Acuña D, Martins TF, Guglielmone AA. Chapter 1 - Tick classification, external tick anatomy with a glossary, and biological cycles. In: Nava S, Venzal JM, González-Acuña D, Martins TF, Guglielmone AA, editors. Ticks: Biology, Ecology, and Control. Academic Press. 2017;1–23. Available from: http://dx.doi.org/10.1016/B978-0-12-811075-1.00001-7
- Vashakidze E, Megrelishvili T, Mikadze I, Kalandadze I, Gegeshidze T, Pachkoria E, et al. Tick-borne zoonotic infection in Georgia. Int J Infect Dis. 2018;73:393. Available from: http://dx.doi.org/10.1016/j.ijid.2018.04.4305
- Doss MA. Ticks and tickborne diseases: Geographical distribution of ticks. U.S. Government Printing Office. 1974. Available from: https://books.google.ge/books?id=RJ0NAAAAIAAJ
- Tsapko NV. The tick Ixodes kaiseri (Acari, Ixodidae) in the North Caucasus and Transcaucasia according to the material from the collection of Stavropol Anti-Plague Institute. Entomol Rev. 2018;98:125–128. Available from: http://dx.doi.org/10.1134/S0013873818010116
- European Centre for Disease Prevention and Control. Tick maps. ECDC. 2020. Available from: https://www.ecdc.europa.eu/en/disease-vectors/surveillance-and-disease-data/tick-maps
- Stepanović S, Kosovac A, Krstić O, Jović J, Toševski I. Morphology versus DNA barcoding: two sides of the same coin. A case study of Ceutorhynchus erysimi and C. contractus identification. Insect Sci. 2016;23(4):638-48. Available from: https://doi.org/10.1111/1744-7917.12212
- Ondrejicka DA, Morey KC, Hanner RH. DNA barcodes identify medically important tick species in Canada. Genome. 2017;60:74–84. Available from: https://doi.org/10.1139/gen-2015-0179
- Zhang RL, Zhang B. Prospects of using DNA barcoding for species identification and evaluation of the accuracy of sequence databases for ticks (Acari: Ixodida). Ticks Tick Borne Dis. 2014;5:352–358. Available from: https://doi.org/10.1016/j.ttbdis.2014.01.001
- Krčmar S, Klobučar A, Vucelja M, Boljfetić M, Kučinić M, Madić J, et al. DNA barcoding of hard ticks (Ixodidae), notes on distribution of vector species and new faunal record for Croatia. Ticks Tick Borne Dis. 2022;13:101920. Available from: https://doi.org/10.1016/j.ttbdis.2022.101920
- Evans M. Molecular barcoding of Australian ticks. Murdoch University; 2018. Available from: https://researchrepository.murdoch.edu.au/id/eprint/42887/1/Evans2018.pdf?fbclid=IwAR1b0zvIUF2-GsyIbvjAT1Dflku5SxKbNNW0XFZuQfenhAzdwiOnSSlWPk4
- Bouchard C, Dumas A, Baron G, Bowser N, Leighton PA, Lindsay LR, et al. Integrated human behavior and tick risk maps to prioritize Lyme disease interventions using a ‘One Health’ approach. Ticks Tick Borne Dis. 2023;14:102083. Available from: https://doi.org/10.1016/j.ttbdis.2022.102083
- Stoeckle M. Taxonomy, DNA and the barcode of life. BioScience. 2009;53:796–797. Available from: https://doi.org/10.1002/9781119654803.ch10
- Ratnasingham S, Hebert P. BOLD: The Barcode of Life Data System (www.barcodinglife.org). Mol Ecol Notes. 2007;7:355–364. Available from: https://doi.org/10.1111/j.1471-8286.2007.01678.x
- Estrada-Peña A, Mihalca A, Petney T. Ticks of Europe and North Africa: A Guide to Species Identification. Available from: https://link.springer.com/book/10.1007/978-3-319-63760-0
- Folmer O, Black M, Wr H, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial Cytochrome C oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol. 1994;3:294–299. Available from: https://pubmed.ncbi.nlm.nih.gov/7881515/
- Barrett R, Hebert P. Identifying spiders through DNA barcodes. Can J Zool. 2005;83:481–491. Available from: http://dx.doi.org/10.1139/z05-024
- Zhang Z, Schwartz S, Wagner L, Miller W. A greedy algorithm for aligning DNA sequences. J Comput Biol. 2000;7:203–214. Available from: https://doi.org/10.1089/10665270050081478
- Ratnasingham S, Hebert PDN. A DNA-based registry for all animal species: The Barcode Index Number (BIN) system. PLoS One. 2013;8(7):e66213. Available from: https://doi.org/10.1371/journal.pone.0066213
- Zhang J, Kapli P, Pavlidis P, Stamatakis A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics. 2013;29(22):2869–2876. Available from: https://doi.org/10.1093/bioinformatics/btt499
- Zakalashvili M. The Results are published in NCBI GenBank and BOLD Dataset (Dataset ID: DS-1495). BOLD Systems. 2023. Available from: https://dx.doi.org/10.5883/DS-1495
- Chan A, Chiang L-P, Hapuarachchi HC, Tan C-H, Pang S-C, Lee R, et al. DNA barcoding: complementing morphological identification of mosquito species in Singapore. Parasites Vectors. 2014;7:569. Available from: https://doi.org/10.1186/s13071-014-0569-4
- Sheth BP, Thaker VS. DNA barcoding and traditional taxonomy: an integrated approach for biodiversity conservation. Genome. 2017;60:618–628. Available from: https://doi.org/10.1139/gen-2015-0167
- Jones AM, Van De Wyngaerde MT, Machtinger ET, Rajotte EG, Baker TC. Choice of laboratory tissue homogenizers matters when recovering nucleic acid from medically important ticks. J Med Entomol. 2020;57:1221–1227. Available from: https://doi.org/10.1093/jme/tjaa006
- Young MR, deWaard JR, Hebert PDN. DNA barcodes enable higher taxonomic assignments in the Acari. Sci Rep. 2021 Aug 5;11(1):15922. Available from: https://doi.org/10.1038/s41598-021-95147-8
- Gharbi M, Darghouth MA. A review of Hyalomma scupense (Acari, Ixodidae) in the Maghreb region: from biology to control. Parasite. 2014;21:2. Available from: https://doi.org/10.1051/parasite/2014002
- Walter M, Brugger K, Rubel F. The ecological niche of Dermacentor marginatus in Germany. Parasitology Res. 2016;115:2165–2174. Available from: https://doi.org/10.1007/s00436-016-4958-9
- Saari S, Näreaho A, Nikander S. Chapter 9 - Arachnida. In: Saari S, Näreaho A, Nikander S, editors. Canine Parasites and Parasitic Diseases. Academic Press; 2019. p. 187–228. Available from: https://doi.org/10.1016/B978-0-12-814112-0.00009-X
- CABI. Rhipicephalus bursa. 2022. Available from: https://www.cabi.org/isc/datasheet/65996#topathogensVectored
- CFSPH. Rhipicephalus (Boophilus) annulatus. 2007. Available from: https://www.cfsph.iastate.edu/Factsheets/pdfs/boophilus_annulatus.pdf